What Is the Hardest Color to Create for Fireworks? The Secret Behind the Blue Flame
From National Day to New Year’s Eve, festive celebrations around the world are often lit up by dazzling fireworks. Think back—what colors and effects have you seen in the sky? Red, green, yellow, and silvery white flashes are probably among the most common. But what is the hardest color to create for fireworks? The answer is blue.

Why Is Blue the Most Difficult Color in Fireworks?
Even today, a vivid and pure blue firework remains one of the highlights of any professional fireworks show. In fact, it’s often referred to as the holy grail of the pyrotechnics industry. But why is blue so notoriously hard to produce? To understand that, we first need to take a look at how fireworks display color.
Fireworks don’t use paint or pigments to show color. Instead, they rely on a phenomenon known as the flame test, where different metal compounds emit specific colors when burned. This happens due to a physical process called atomic emission.
When metal elements are heated in a flame, their outer electrons absorb energy and “jump” to higher energy levels—similar to a baseball being hit up into the air. But this elevated state is unstable. The electrons soon return to their original lower-energy states, releasing energy in the form of electromagnetic waves.
The wavelength of this emitted light depends on the specific element, creating the brilliant colors we see. Many metal salts emit wavelengths within the visible light spectrum, which is why fireworks can be so colorful. What we perceive as color is actually the result of millions of atoms releasing energy in a coordinated blue glow.
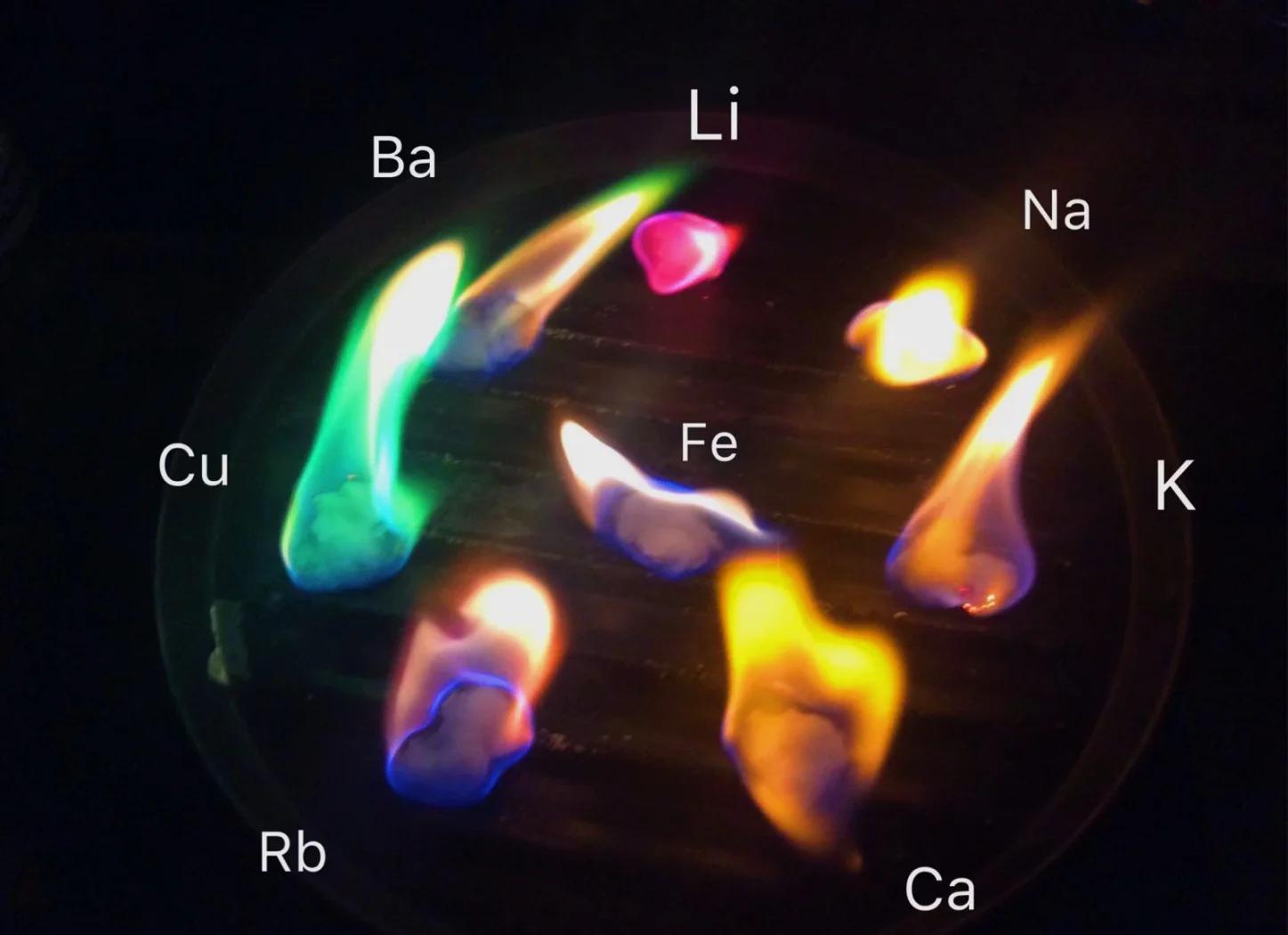
The Chemistry of Blue Fireworks
To create blue fireworks, pyrotechnicians typically use copper or copper compounds, such as copper powder, copper sulfate, copper carbonate, copper nitrate, copper hydroxide, and basic copper carbonate. However, the most vibrant and pure blue is achieved using copper(I) chloride (CuCl), which emits light in the 420–460 nm wavelength range. Other copper salts often produce a greenish-blue tint that lacks the visual clarity of true blue.
Now that we understand the chemistry, let’s return to the central question: What makes blue fireworks so hard to get right?

1. The Color Must Compete with the Night Sky
Although the night sky looks black to the human eye, it actually has a subtle blue hue. Making a blue firework stand out against this background requires extreme precision. Even slight changes in chemical composition can affect both the flame temperature and the resulting color, making it especially hard to produce a clear, vibrant blue that contrasts sharply with the sky.
2. High Temperatures Destroy the Color
There’s also a natural conflict at play. The brighter and more vivid a firework color, the higher the burn temperature usually is. But blue is different. Copper(I) chloride, the compound responsible for that perfect blue, breaks down at relatively low temperatures. If the temperature exceeds about 1200°C, the compound starts to decompose, and instead of blue, you get unwanted white or off-color light. In short, the conditions needed for blue are a narrow target to hit—hot enough to burn, but not so hot that the color is lost.
3. Material Limitations and Cost
The biggest obstacle, however, may be the scarcity of suitable compounds. While there are many metal salts available for red, green, or yellow effects, copper(I) chloride remains almost the only effective source of deep blue. Although some organic copper salts can also generate blue, they’re significantly more expensive and not ideal for large-scale production. Since fireworks require substantial volumes of raw materials, cost is a major factor, especially in mass production, like that seen in China fireworks manufacturing.

A Centuries-Long Search
Pyrotechnic experts have spent centuries looking for an alternative to copper(I) chloride. So far, it’s still the best option. Engineers in China and around the world continue to experiment with different fuel and oxidizer combinations, seeking the perfect formulation that can deliver intense, true blue without sacrificing stability or safety.
Despite the challenges, manufacturers—especially those producing China fireworks for global markets—continue to push the boundaries. They refine formulas, adjust burn rates, and tweak chemical balances to make each burst of blue more striking than the last.

Conclusion
In short, when you ask “What is the hardest color to create for fireworks?”—the answer is undeniably blue. It’s a delicate balance of chemistry, temperature control, and cost-efficiency, and it remains one of the most prized effects in the pyrotechnic world. From the workshops of China fireworks factories to grand international shows, the quest for the perfect blue continues.
