The Science Behind Fireworks: Chemistry, Colors, and Creativity
During festivals and celebrations, fireworks light up the night sky with a dazzling display. As each shell bursts high above, it scatters vibrant sparks across the darkness, transforming the sky into a garden of light. But behind this beauty lies fascinating chemical magic.

What Are Fireworks Made Of?

1. Black Powder (Gunpowder)
At the heart of every firework is black powder, a mixture of potassium nitrate (75%), charcoal (15%), and sulfur (10%). These ingredients react to create explosions, providing the lift and burst effect that propels fireworks skyward.
2. Metal Powders and Chemical Compounds
These components are the secret to the bright colors in fireworks. Sodium produces yellow flames, copper emits green, and strontium gives off red. These colorful effects are achieved through a process known as flame reaction or flame coloration.
3. Additional Additives
Other materials like oxidizers, color enhancers, and binders are used to improve performance. Oxidizers help intensify combustion and color, enhancers boost brightness, and binders hold the powder mixtures together to form pellets that shape the visual effects.

How Fireworks Get Their Colors
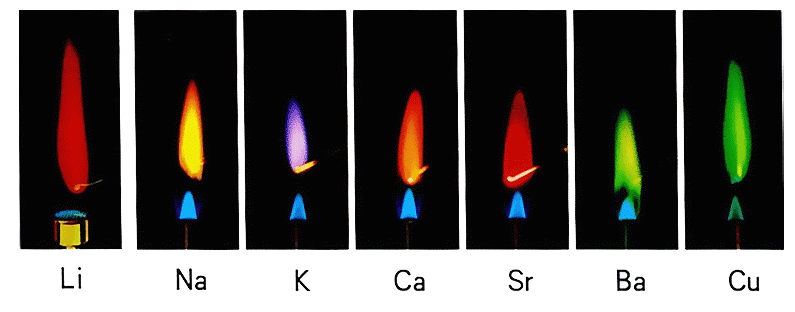
So, how do fireworks display such a rainbow of colors? The answer lies in a chemical process called the flame test or flame coloration. Certain metals or their compounds, when burned in a flame, emit specific colors. For example:
- Calcium burns brick red
- Potassium burns violet
- Sodium burns yellow
By deliberately adding these metal salts into fireworks, designers can create vivid and controlled color effects in the sky.

How Are Fireworks Shapes Created?
Once the colors are solved, what about those unique shapes—hearts, chrysanthemums, tadpoles?

It’s all in the materials and arrangement. For example, adding hardwood charcoal powder can produce golden, sparkling particles. These particles ignite mid-air, creating a star-like shimmer as they burn in oxygen-rich air.
To form complex shapes, designers create small spherical pellets called stars, made of gunpowder, starch, and metal salts. These stars are arranged into specific patterns inside the firework shell. When the shell explodes, the stars ignite in the designed layout, forming recognizable shapes in the sky.

How do Fireworks Go up?
Getting the fireworks into the sky requires a precise launch system. After the firework is assembled, it’s placed into a launch tube filled with a propellant—usually more black powder. Once ignited, the propellant generates intense heat and expanding gases that shoot the shell upward.
As it ascends, a timed fuse ignites the internal bursting charge, lighting the stars and unleashing the full firework effect high in the air.

PyroNexa Fireworks and Environmental Protection
Traditional fireworks can release pollutants like sulfur dioxide, nitrogen oxides, and heavy metals. In response, PyroNexa has developed a line of eco-friendly fireworks. These innovative products use:
- Lead-free catalysts
- Organic cellulose
- Low-metal powder formulas
These improvements significantly reduce harmful emissions, making our fireworks safer for the environment and your audience.

PyroNexa, a trusted China fireworks manufacturer and Chinese fireworks supplier, combines centuries-old craftsmanship with cutting-edge technology to produce stunning yet sustainable pyrotechnics. We strictly adhere to international quality standards and offer a wide range of custom fireworks solutions.
Whether you’re an event planner, importer, wholesaler, or distributor, we offer OEM and private label fireworks tailored to your market needs. Our professional team supports you from concept to shipment, ensuring reliable supply and eye-catching displays.
